
Three domains give us and sp 2 hybridization and so on. Lone pairs and covalent bonds with other atoms contribute to being electron domains. Therefore, the Lewis structure of SeO 2 is given below as: SeO 2 HybridizationĪn easy method to determine the hybridization of an atom in an element is to observe the number of its electron regions or electron domains.
This tells us that the SeO 2 compound forms two double bonds with Oxygen to obtain its most stable structure. Upon calculating the formal charges, we see that they come up to zero. The structure is then modified as shown below: The presence of a positive +1 charge indicates the need for another double bond. The presence of positive and negative formal charges tells us that this may not be the most stable structure for SeO 2. In this case, the formal charges are given by: Element V N B/2 FC O 6 6 2/2 -1 S 6 2 6/2 +1 O 6 4 4/2 0 It is determined such that the elemental charge on each atom is closest to zero.įC = Valence Electrons – Non-bonding electrons – (Bonding electrons ÷ 2) To verify if the above structure is stable, we need to calculate the formal charges on each of the atoms.įormal charges for an element/structure help determine its most stable Lewis Structure state. This tells us that we need to move one of the outer lone pairs of valence electrons inward. The remaining two valence electrons go to Selenium and act as a lone pair.Īs shown above, the central Se does not have an octet as it has only six valence electrons. The two oxygen atoms fulfill their electron requirements in accordance with the octet rule. The remaining valence electrons are placed on the outermost or most electronegative atoms first. The valence electrons are first placed between the Selenium and Oxygen atoms to form covalent bonds. The two oxygen atoms are placed alongside it in the skeletal structure, as shown in the figure. Selenium is the least electronegative and will act as the central atom. Thus, the total number of valence electrons in Selenium Dioxide is given by:Ħ + 12 = 18 valence electrons. Therefore, two Oxygen atoms contribute 6 x 2 = 12 valence electrons. Oxygen’s electronic configuration is 1s 2 2s 2 2p 4. Therefore, a single atom of Selenium contributes 1×6 = 6 valence electrons.īeing in group 6 of the periodic table, Oxygen has six valence electrons and has a valency of -2. Selenium belongs to group 16 in the periodic table and has an electronic configuration of 4s 2 3d 10 4p 4. To calculate the total number of valence electrons present, we need to identify the valence electrons each element can contribute to the molecule. SeO 2 comprises of one selenium atom and two atoms of Oxygen.

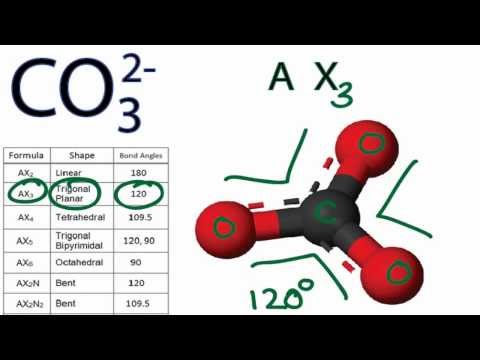
Looking at the molecular geometry of SO2, the bond angle is at 120°. There is polarization because Sulfur pulls the charge of the molecule to its side while gaining partial negative charge and not Oxygen since it is the least electronegative atom, making SO2 a polar molecule. There is an imbalance charge across other atoms in the molecule, making Sulfur Dioxide polar. With SO2, the pairs of bonding electrons are arranged at an angle of 120 degrees. Electron geometry includes all the pairs of electrons, even the lone pairs. This is not similar to molecular geometry, where only the total number of atoms is considered to determine its shape. The electron geometry of SO2 is trigonal planar.


 0 kommentar(er)
0 kommentar(er)
