
Nucleus and I'll just draw their p orbitals. Two atoms, and I'll just draw one of each of their Kind of in the direction that they're pointing? And the other type of bond youĬould have, you can imagine if you have two p orbitals. Kind of bond could there be where my two orbitals overlap So this right here- let me make this clear. There be any other type of bond than that? Well, the other type of bond, There's an overlap kind of in the direction in which the That's the small lobe,Īnd then that's the big lobe like that. Hybridized orbital, and that's on this atom and this is kind of Me draw two nucleuses and let me just draw one When we draw tetrahedral geometries of sp3 carbons like that found in methane it is conventional to draw two bonds in the plane of the page (straight or solid lines), one bond behind the plane as a dashed line, and the fourth bond as a shaded triangle coming out of the plane. Tetrahedral geometry creates a tetrahedron which is a four-faced triangular pyramid with bond angles of 109.5 degrees between each of the hydrogens. To minimize these repulsions between the hydrogens the methane adopts a tetrahedral geometry. These hydrogen atoms each have electron clouds around them which are negative and repel each other.

An sp3 hybridized carbon like methane has four bonds each going to a single hydrogen atom. And a dashed line means a bond going away from you into the plane of the page. A shaded triangle (or wedge) means a bond coming toward you out the plane of the page. A straight line (or solid line) represents a bond that is part of the plane of the page. It's meant to show the 3-D shape of bonds in molecules like the sp3 hybridized bonds in methane. They can overlap, but in different ways, and the bonds thus formed are not called sigma bonds but pi bonds. Since the other orbitals are not oriented along the bond axis, they cannot overlap "head on". pz orbital, along the z axis, and any s orbital (which is spherical) can overlap to form a sigma bond. If the z-axis is taken as the bond axis, only orbitals with the central axis along z-axis can form sigma bond. Head on overlap is actually a layman's term to specify the requirement of specific symmetries in combining atomic orbitals.

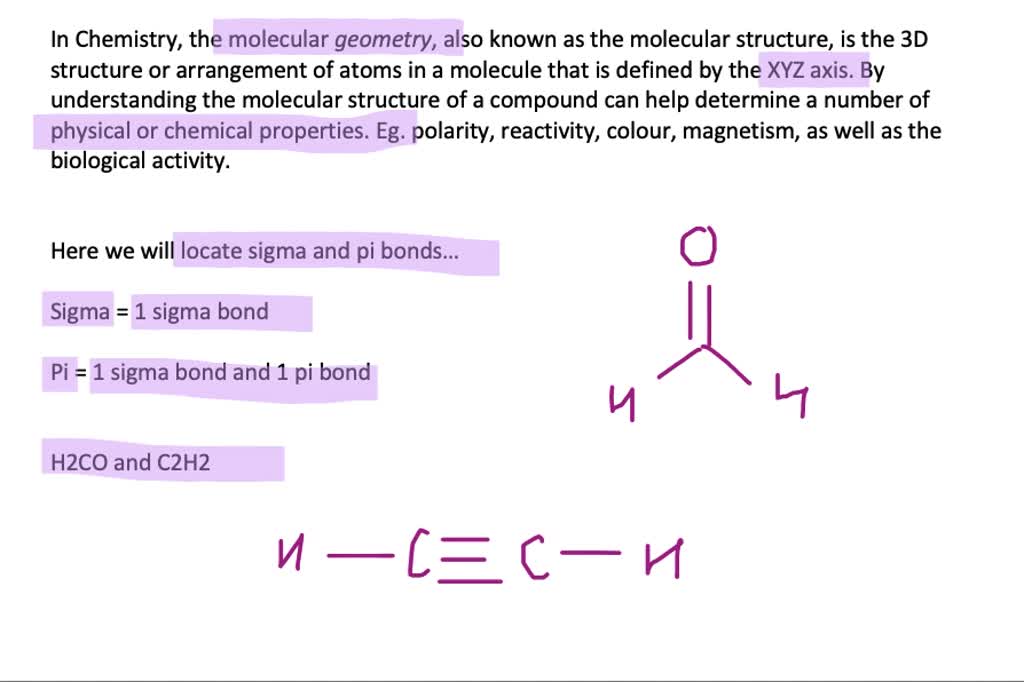
A sigma bond involves head on overlap of atomic orbitals. The second question can be much more satisfactorily answered. In that concept, there is no explanation as to why we do not include the inner orbitals, but by not including them we get the right answers, and hence that became a so called "rule" of hybrid orbitals. Actually, there are no hybrid orbitals and hybridisation concept, introduced by Pauling is now obsolete and replaced with the superior molecular orbital model, which answers all the shortcomings of hybridisation, one of which you just mentioned. A god question, but unfortunately no simple answer. An example of sp hybridisation is ethyne, C 2H 2. Carbon undergoes sp hybridisation when it forms a triple bond, creating two equal orbitals. Sp hybridisation forms a linear structure with 180° between the orbitals. An example of sp 2 hybridisation is ethene, C 2H 4. Carbon undergoes sp 2 hybridisation when it forms a double bond, producing three equal orbitals.

Sp 2 hybridisation forms a triangular planar shape with 120° between the orbitals. An example of sp 3 hybridisation is methane, CH 4. Carbon undergoes sp 3 hybridisation when it forms four single bonds and produces four equal orbitals. Sp 3 hybridisation forms a tetrahedral structure with 109.5° between the orbitals. There are three types of hybridisation: sp 3, sp 2 and sp. A double bond contains one sigma bond and one pi bond, whereas a triple bond contains one sigma bond and two pi bonds. Pi bonds occur when two pi orbitals overlap sideways and they only form within a double or triple bond. Sigma bonds occur when two atomic orbitals overlap along the bond axis and this bond always forms in a single covalent bond. When atomic orbitals overlap they form two types of covalent bonds: sigma and pi. The atom can form stronger covalent bonds using these hybrid orbitals. A hybrid orbital results from the mixing of different atomic orbitals on the same atom.


 0 kommentar(er)
0 kommentar(er)
